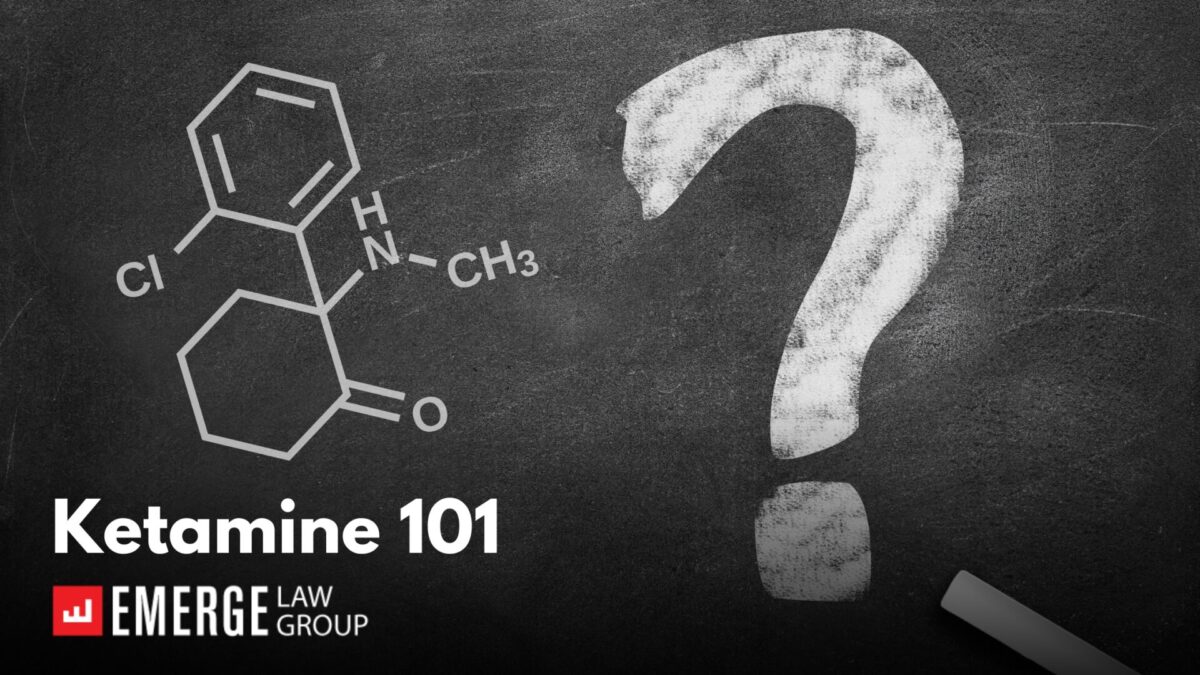
Author: Matt Brockmeier, Of Counsel
While much of our work in the Psychedelics Practice Group at Emerge Law Group involves the Oregon Psilocybin Services Act or the Colorado Natural Medicine Health Act, as well as similar efforts in other states, we also get a lot of questions about ketamine, the only “legal” psychedelic in most of the US. Because drug policy and chemistry are complicated there are a lot of misconceptions about ketamine – the legality of the drug itself, the new therapies and treatments that have proliferated around it, and whether it is in fact a horse tranquilizer. Here is what you need to know about ketamine.
Is ketamine a horse tranquilizer?
No, and it’s not a psychedelic. Well, maybe. It depends on who you ask. While also used in veterinary procedures, ketamine is an FDA-approved synthetic non-barbiturate dissociative anesthetic first synthesized in 1962 by a chemist for Parke-Davis looking for an alternative to the potent hallucinogenic phencyclidine, or PCP.[1] If you’ve had surgery under general anesthesia there’s a good chance you’ve used ketamine.
Notwithstanding reports that the tragic recent death of actor Matthew Perry involved ketamine, it actually has a much more favorable safety profile compared to other anesthetics because it does not depress breathing or blood pressure .[2] It is easy to administer and is an ideal anesthetic for patients who have lost a lot of blood or who have dangerously low blood flow. It is also often the only available anesthetic in rural areas of developing countries and in combat situations.[3] In fact, ketamine is on the World Health Organization’s List of Essential Medicines for Anesthesia and Pain Management. And in recent years Ketamine has emerged as a tool in mental health as a treatment for conditions like depression, anxiety, and PTSD.
So is it approved for depression?
Not exactly. While ketamine has shown incredible promise in clinical trials for treating a variety of mental health conditions and is currently being used around the world by a growing number of doctors and clinicians to treat multiple mental health conditions, it was not approved to treat depression. Or PTSD. Or anxiety. It was approved as a general anesthetic.[5] Ketamine’s antidepressant properties weren’t discovered until much later.
Traditionally a medication is prescribed for a specific use, as approved by the FDA, which is on the drug’s label. However, the practice of “off-label” prescribing involves using drugs for purposes beyond their approved indications. This approach allows healthcare providers to explore innovative solutions for conditions that may be resistant to conventional treatments or therapies. Estimates for how common off-label prescriptions are in the United States are hardly precise, but it is estimated that well over 20% of all drugs are prescribed for reasons other than those specified in the FDA approval and labeling.[6]
But how can a doctor prescribe a drug for something it’s not approved for?
There is a common misconception that the FDA regulates prescription drugs, but the administration’s legal authority is more proscribed. Pursuant to the Food, Drug and Cosmetic Act of 1938 and subsequent amendments, FDA oversees the production, sale, and distribution of food, drugs, medical devices, and cosmetics in interstate commerce.[7] Regarding pharmaceuticals, FDA can basically approve, deny or recommend the recall of a prescription drug.[8] It also regulates prescription drug labeling and advertising, and can grant periods of exclusivity to new drugs.[9] But once a drug has been approved for a certain use, FDA has little control over how it is prescribed. That is because drugs are prescribed by doctors, and doctors are regulated at the state level.
State medical boards regulate the practice of medicine, including prescribing drugs, usually under authority of a state Medical Practice Act conferring prescribing authority. In a rare departure from this approach, the FDCA does impose criminal penalties on physicians prescribing human growth hormone off label.[10] But historically and legally, decisions about medication are between the doctor and the patient, not the FDA (or DEA). As long as the prescriber possesses the appropriate licenses, if they determine that it is in the patient’s best interest and consistent with prevailing standard of care, they may prescribe a drug for an indication not on the label. Moreover, Medicare, Medicaid, and private insurers can and do reimburse for off-label uses when there is some evidence to support such uses.
So, it’s legal to buy, sell, and use a drug off- label?
Not so fast. Under the Controlled Substances Act, which is enforced by the DEA, it is illegal to import, manufacture, distribute, possess, or use a controlled substance without a valid prescription. There are five Roman-numerated Schedules (I-V) under the CSA. A drug’s scheduling is based on its accepted medical use, potential for abuse, and safety or dependence liability. With respect to pharmaceutical controlled substances, DEA’s statutory responsibility is twofold: to prevent diversion and abuse of these drugs while ensuring an adequate and uninterrupted supply is available to meet the country’s legitimate medical, scientific, and research needs. Ketamine is a Schedule III drug, which means that while it has an accepted medical use it also has a moderate to low potential for physical and psychological dependence.[11] To prescribe it (and other controlled substances) doctors and clinicians must register with the DEA. But despite being approved as an anesthetic, a doctor with a valid state medical license and DEA registration can legally prescribe it for another indication based on emerging science or current medical consensus. A patchwork of prescriber and clinician codes of conduct and scopes of practice intersect and overlap with state and federal laws in this area, the legality of something often depends on the specific jurisdiction, substance, and licenses. The patient may only use it as prescribed, or risk violating state and federal law. And then it is legal to dispense, prescribe and use.
Will ketamine be approved for depression?
Possibly. Ketamine was first patented in the US in 1966. FDA approved ketamine (under the brand name Ketalar) in 1970 as an anesthetic.[12] And it wasn’t scheduled on the Controlled Substances Act until 1999.[13] Its patent has long since expired, so other manufacturers sell generic versions of the same drug under a different name. Now it is manufactured by multiple companies around the world and is generally readily available. However, to obtain FDA approval for depression (or any new condition for that matter), someone must conduct lab, animal, and human clinical testing and then submit that trial data to FDA to demonstrate safety and efficacy for that new condition. In one study the median total cost of the clinical trials required by FDA for a drug was estimated at $48 million.[14]
Normally a drug developer sets out to create a new drug to treat a disease or condition and takes that molecule through clinical trials so that it can be sold as a prescription medication, and then applies for a patent for that drug to protect its investment in the form of intellectual property. The USPTO grants patents for new drugs, which it can do any time during a drug’s development as a pharmaceutical.[15] But ketamine’s patent has expired and is not eligible for renewal, so there is little economic incentive for a company to take ketamine through clinical trials.
Generic ”racemic” ketamine is a mixture of two enantiomers that are mirror images of each other, S-ketamine (esketamine) and R-ketamine (arketamine). Think of the left and right hands of a glove. Researchers first demonstrated ketamine’s therapeutic effects on depression about 20 years ago. Primarily for this reason the pharmaceutical company Johnson & Johnson took esketamine – the left glove – through trials for depression. They were successful. In 2019, 19 years after peer-reviewed trials showed ketamine’s potential as a mental health medication, FDA approved the use of intranasal esketamine under the brand name Spravato for the treatment of drug-resistant depression.[16] As late as last year a company called Perception Neurosciences, owned by Atai Life Sciences, was developing PCN-101 ( arketamine, or the other glove) for treatment-resistant depression, but it is still in development as of this writing. If you feel like this isn’t exactly the sort of innovation the patents are supposed to encourage or protect, you are not alone. There are many who feel these drugs represent manipulation of the patent process and an example of why our larger patent system needs reform.
Spravato/Esketamine enjoys both a patent granted by the USPTO, and exclusivity conferred by FDA, which means that no competitors can make or sell it for a period of time. But it is also subject to what is known as a risk evaluation and mitigation strategy. A REMS is a required risk management plan that can include one or more elements to ensure that the benefits of a drug outweigh its risks. In the case of Spravato, due to the risks of sedation or loss of consciousness, dissociation, respiratory depression, and abuse, it requires supervision and training.[17]
Will insurance pay for it?
That depends. Ketamine is relatively inexpensive and very common today, but until recently most insurance companies would not cover it for therapeutic or antidepressant use off label. That’s changing, though, as at home ketamine telehealth companies and ketamine clinics work to create innovative payment and delivery solutions in compliance with – but sometimes testing the boundaries of – state and federal laws, rules, and regulations.
What your insurance covers depends on a lot of things, not the least of which includes specific policy language but also state law and claims filing requirements. FDA has issued several warnings in the past few years, primarily about the unmonitored use of compounded ketamine, but under certain circumstances, such as the establishment of a proper doctor-patient relationship, adequate safety screening and dosage protocols, and the appropriate monitoring or supervision, insurance can legally cover ketamine. Recently the American Medical Association approved three Current Procedural Terminology or CPT Codes related to psychedelic therapy.[18] CPT codes are used to standardize billing for medication and treatments and to provide coverage and reimbursement for those therapies.
So ketamine is the only legal psychedelic, right?
Well yes and no. It’s not technically a psychedelic, or at least not a “classic” serotonergic psychedelic like LSD, DMT, psilocybin, because it doesn’t act on our serotonin receptors. It is an N-methyl-D-aspartate (NMDA) antagonist that acts on the gamma-aminobutyric acid (GABA)-ergic and glutamatergic systems. And it’s not really the only legal one, it’s just the only one legal at the federal level. Psilocybin is legal in Oregon and Colorado and possibly in several other states in the next few years. And soon enough it may be approved at the federal level. The recent HHS recommendation to deschedule cannabis and the pending approval of MDMA may represent just the beginning of more comprehensive reform of the Controlled Substances Act. We will leave that for another post. But in a growing number of towns and cities across the states and the world, under the right conditions with medical attention/ supervision, and in compliance with layers of laws and regulations to ensure safety and prevent abuse, ketamine is already being used legally, off-label, to treat a number of mental health conditions.
Conclusion
Remember, FDA regulates the introduction, sale, marketing, and advertising of prescription drugs under the Food, Drug and Cosmetics Act. USPTO grants patents. DEA enforces the Controlled Substances Act. Doctors prescribe medicine. Insurance companies decide what gets paid for. And ketamine is not a horse tranquilizer.
If you have any questions, don’t hesitate to contact lawyers from our psychedelics group regulatory team: Dave Kopilak, Sean Clancy, Alex Berger, Kaci Hohmann, and Matt Brockmeier.
[1] Mion, Georges. (2017). History of anaesthesia: The ketamine story – past, present and future. European Journal of Anaesthesiology. 34. 1. 10.1097/EJA.0000000000000638.
[2] WHO Fact file on ketamine, March 2016 (https://sa1s3.patientpop.com/assets/docs/55947.pdf)
[3] Mercer SJ. ‘The Drug of War’–a historical review of the use of Ketamine in military conflicts. J R Nav Med Serv. 2009;95(3):145-50. PMID: 20180434.
[5] Mion, Georges. (2017). History of anesthesia: The ketamine story – past, present and future. European Journal of Anesthesiology. 34. 1. 10.1097/EJA.0000000000000638.
[6] Van Norman GA. Off-Label Use vs Off-Label Marketing of Drugs: Part 1: Off-Label Use-Patient Harms and Prescriber Responsibilities. JACC Basic Transl Sci. 2023 Feb 27;8(2):224-233.
[7] Wax PM. Elixirs, diluents, and the passage of the 1938 Federal Food, Drug and Cosmetic Act. Ann Intern Med. 1995 Mar 15;122(6):456-61.
[8] 21 C.F.R. § 7 et seq.
[9] 21 C.F.R. 314.108, 316.31, 316.34 and sections 505A, 505E, and 505(j)(5)(B)(iv) of the FD&C Act.
[10] 21 U.S.C. § 396 (2000). See also 21 C.F.R. § 312.2(d) (2008).
[11] 21 U.S.C. 801 et seq.
[12] Li L, Vlisides PE. Ketamine: 50 Years of Modulating the Mind. Front Hum Neurosci. 2016 Nov 29;10:612.
[13] 21 CFR Part 1308.
[14] Moore TJ, Heyward J, Anderson G, Alexander GC. Variation in the estimated costs of pivotal clinical benefit trials supporting the US approval of new therapeutic agents, 2015-2017: a cross-sectional study. BMJ Open. 2020 Jun 11;10(6):e038863.
[15] Frequently Asked Questions on Patents and Exclusivity, FDA. https://www.fda.gov/drugs/development-approval-process-drugs/frequently-asked-questions-patents-and-exclusivity
[16] “FDA approves new nasal spray medication for treatment-resistant depression; available only at a certified doctor’s office or clinic” FDA Press Release, March 05, 2019.
[17] Id.
[18] “MAPS PBC Announces New American Medical Association CPT III Codes for Psychedelic-Assisted Therapies Take Effect”